Copper Ii Sulfate and Mercury I Nitrate
See the answer See the answer See the answer done loading. Scrap iron is then added to this solution and pure copper metal precipitates out because of the following chemical reaction.

Oneclass Write Net Ionic Equations For The Reaction If Any That Occurs When Aqueous Solutions Of T
Strontium nitrate and potassium iodide.
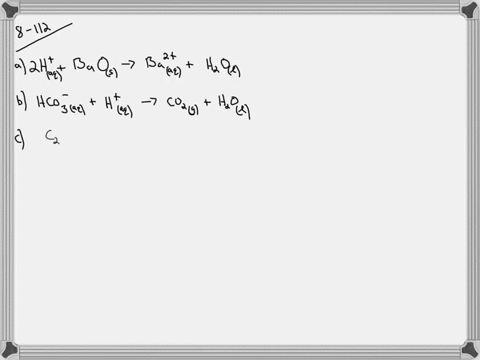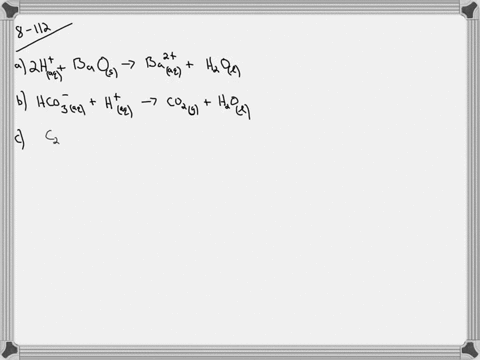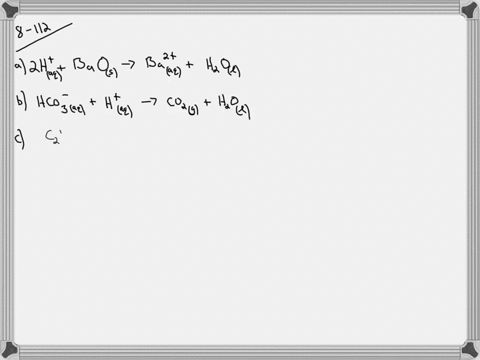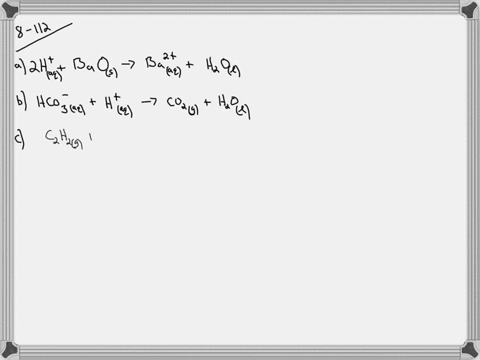
. Fes CuSO4aq â Cus FeSO4aq Suppose an industrial quality-control chemist analyzes a sample from a. CopperII sulfate and mercuryI nitrate d. Um Which well find out in a minute.
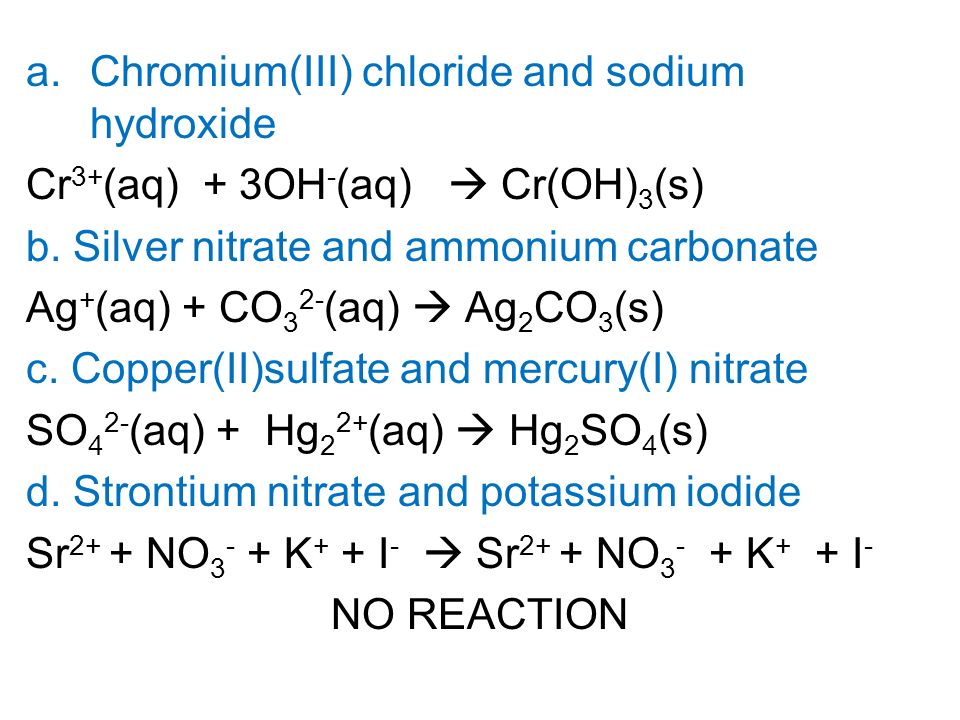
Chromium III chloride and sodium hydroxide. Write the net iconic equation if any that occurs when aqueous solutions of. Copper and Silver Nitrate- Mike Liu Objective- To determine if the copper reacted was copper I or copper II ProcedureWeigh a Packet containing silver nitrate and transfer the AgNo3 into a small 50 mL beaker containing 15 ml of distilled water.
MercuryII nitrate ammonium sulfide mercuryII sulfide ammonium nitrate 2 5. This problem has been solved. Um Okay so copper copper sulfate.
The mercury one nitrate. Now there is something a little bit tricky about the night. Solutions of silver nitrate and sodium chromate are mixed.
CopperII sulfate and mercuryI nitrate. Cus Cu 2 aq 2e-The net effect. Up to 256 cash back c.
Here copper II sulfate CuSO 4 is added to copper II nitrate Cu NO 3 2. Aluminum hydrochloric acid aluminum chloride hydrogen 2Al 6HCl 2AlCl 3 3H 2 41. Copper II sulfate and mercury I nitrate.
The result is no visible reaction. Dilute hydrochloric acid is added to a dilute solution of mercuryI nitrate. Silver nitrate and ammonium carbonate.
Ammonium sulfate and barium nitrate b. So copper too has a too positive self is a tu minus. Copper silver nitrate copperII nitrate silver 4 3.
CopperII nitrate water hydrogen nitrate copperII. A net ionic equation can be predicted from a chemical reaction if it produces a weak electrolyte or non-electrolyte. A solution of copperII sulfate is added to a solution of barium hydroxide.
Here copper II nitrate Cu NO 3 2 is added to copper II sulfate CuSO 4. LeadII nitrate and sodium chloride c. Get 1 free.
Copper II sulfate and mercury I nitrate. MercuryI nitrate and copperII sulfate Complete the reaction below with your response starting with the cation as the first substance. Write net ionic equations for the reaction if any that occurs when aqueous solutions of the following are mixed.
Copper II sulfate iron iron II sulfate copper CuSO 4 Fe FeSO 4 Cu 40. All that happens is a transfer of copper from the anode to the. Strontium nitrate and potassium iodide.
ChromiumIII chloride and sodium hydroxide b. Write net ionic equations for the reaction if any that occurs when aqueous solutions of the following are mixed. 2Ag CrO₄² -- Ag₂CrO₄.
Mercury does not replace copper in a reaction between copper II sulfate and mercury because mercury is below copper in the reactivity level of the periodic table. Science Chemistry QA Library Write the net iconic equation if any that occurs when aqueous solutions of. Water hydrogen oxygen 4 7.
Question 9 1 pts What is the net ionic equation for the reaction between the reagents in aqueous solution shown below. Um Mercury one is a one positive but nitrate is a one negative. The copperII sulfate solution is unchanged.
In this reaction we have lead II nitrate and copper II sulfate. Copper II sulfate and mercury I nitrate are mixed. Strontium nitrate and potassium iodide Question.
For every copperII ion discharged at the cathode a new one is released into solution at the anode. Zinc copperII sulfate zinc sulfate copper 0 4. Okay so we have um cop copper um to sulphate.
And since our Polly atomic sulfate ion usually takes a minus two charge of the formula is just see us. Calcium bicarbonate calcium hydroxide calcium carbonate water CaHCO 3 2 CaOH 2. Predicting Net Ionic Equation.
Up to 256 cash back The sulfuric acid reacts with the copperII carbonate to produce a blue solution of copper II sulfate. Silver nitrate and ammonium carbonate c. Hg2 Select Select Select 1 - Select.
Potential products obtained by swapping the ions are lead II sulfate and. Instead copper atoms in the electrode release electrons and go into solution as Cu 2 aq ions. The result is no visible reaction.
So in C we have copper to sulfate and mercury one nitrate. Carbon oxygen carbon dioxide C O 2 CO 2 42. Write net ionic equations for the reaction if any that occurs when aqueous solutions of the following are mixed.
So the two and copper to solve it tells us the charge on our copper ion s o the charges plus two. Lab in chemistry. Its kind of a weird.
For unlimited access to Homework Help a Homework subscription is required. Silver nitrate reacts with. IronIII hydroxide ironIII oxide water 5 6.
Copper II sulfate and mercury I nitrate.
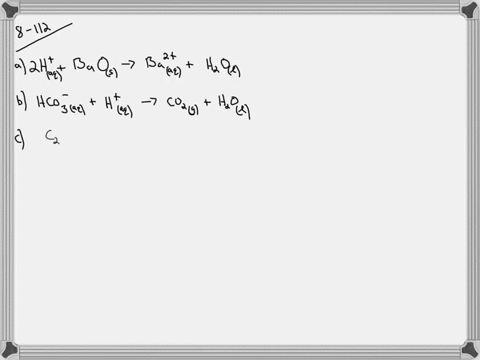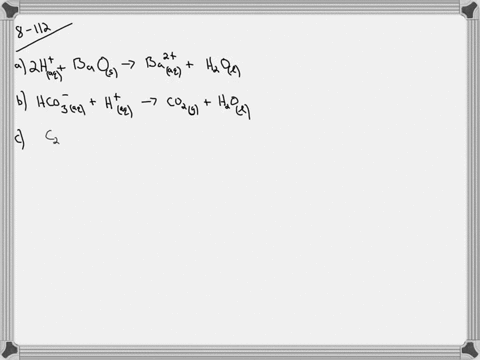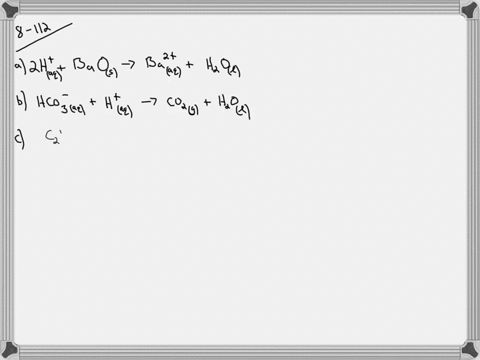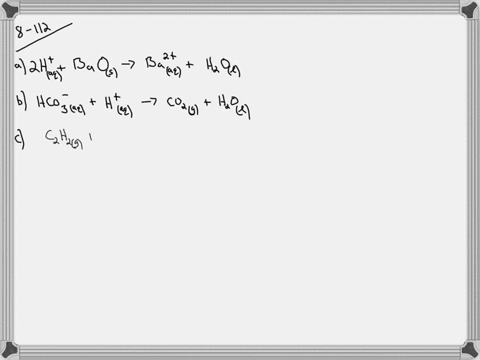
Solved Write Net Ionic Equations For The Reaction If Any That Occurs When Aqueous Solutions Of The Following Are Mixed A Chromium Iii Chloride And Sodium Hydroxide B Silver Nitrate And Ammonium Carbonate C
2nabr Aq Pb Oh 2 Aq Pbbr2 S 2naoh Aq Ppt Video Online Download


Comments
Post a Comment